Table of Contents
Conversion
Discover how conversion transforms yellowcake into uranium hexafluoride (UF₆), ready for enrichment.
What is conversion?
In the Uranium Conversion stage, yellowcake (U3O8) powder is dissolved in nitric acid (HNO3) and converted to uranium hexafluoride (UF6) or, previously, uranium metal (U). UF6 is commonly known as “hex”.
How is conversion carried out?
Step 1: Dissolution
Yellowcake (U3O8) powder, the product of the milling and refining stages of the nuclear fuel cycle, is dissolved in nitric acid (HNO3) to form an aqueous solution.

Step 2: Reduction
Hydrogen is used to reduce the aqueous solution of U3O8 (and UO3) to uranium dioxide (UO2):
U3O8 + 2 H2 → 3 UO2 + 2 H2O

Step 3: Kiln Reaction
Uranium dioxide (UO2) is reacted with hydrofluoric acid (HF) to form uranium tetrafluoride (UF4):
UO2 + 4 HF → UF4 + 2 H2O

Step 4: Fluidised Bed Reaction
Uranium tetrafluoride (UF4) is fed into a fluidised bed with fluorine gas (F2) to form hex (UF6):
UF4 + F2 → UF6
This marks the end of the nuclear fuel cycle’s conversion stage: the hex product is ready for the next stage of the nuclear fuel cycle: enrichment.
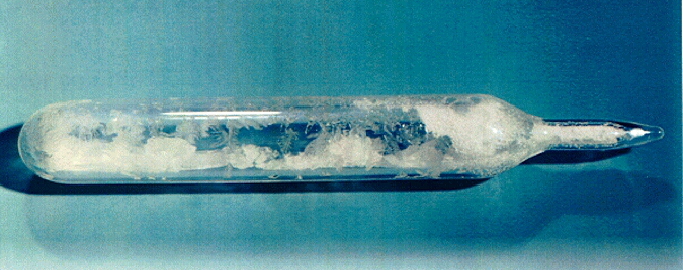
Why is conversion necessary?
Advantages of uranium hexafluoride (UF6), or “hex”, include…
Physical Form
Hex is a crystalline solid at room temperature – ideal for transportation.
Temperature Dependence
Hex sublimes to a gas at a relatively low temperature, 56.5 °C – ideal for enrichment processes.
Pressure Dependence
At high pressures, hex has a liquid phase – ideal for being pumped around chemical processes.
Properties of Fluorine
Fluorine is mono-isotopic – ideal for enrichment processes.
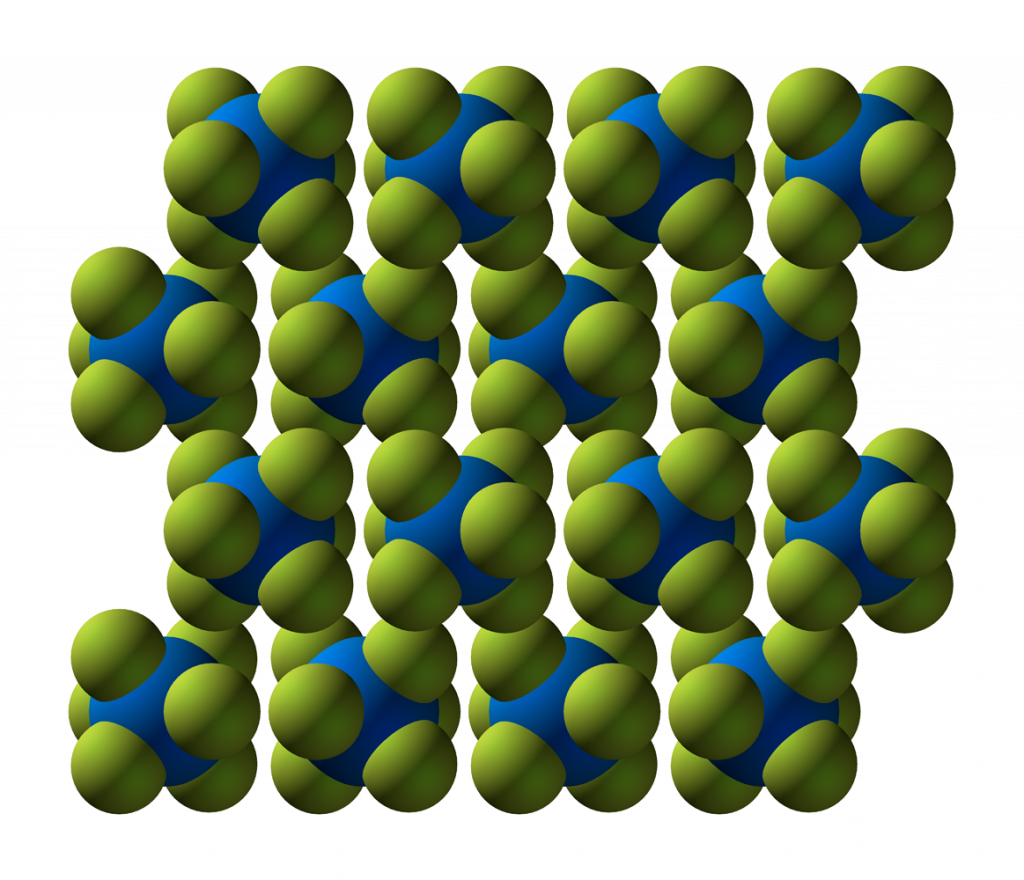
Where does conversion take place?
The conversion stage of the nuclear fuel cycle requires specialist facilities, primarily in countries with large nuclear sectors. Key locations include Canada, China, France, Russia and USA.
Though taking place historically, conversion activities within the UK have now been phased out.

A sealed gas ampoule, containing uranium hexafluoride crystals. This is the final product of the conversion stage.
Explore Further
Choose from the articles below to continue learning about nuclear.
Nuclear Fuel Cycle – Disposal
Uranium Conversion Explained: Turning up the Heat
Nuclear Fuel Cycle – Enrichment
Uranium – An Indispensable Part of Nuclear Energy
Did you know? Explore Nuclear also offers great careers information and learning resources.
Below you can find references to the information and images used on this page.
Content References
- IAEA – Nuclear Fuel Cycle
- NSAN – Graduate Awareness in Nuclear (GAIN) Courses
- Nuclear-Power.com – Uranium Conversion
- US NRC – Stages of the Nuclear Fuel Cycle
- World Nuclear Association – Conversion and Reconversion
- World Nuclear Association – Nuclear Fuel Cycle Overview
- World Nuclear News – A guide: Uranium and the nuclear fuel cycle
Image References
- Yellowcake Barrels, IAEA Imagebank – CC BY-SA 2.0
- Yellowcake, IAEA Imagebank – CC BY-SA 2.0
- Uranium Dioxide Powder, Chemolunatic – CC BY-SA 4.0
- Uranium Tetrafluoride Powder, Chemolunatic – CC BY-SA 4.0
- Uranium Hexafluoride Ampoule, Argonne National Laboratory – Public Domain
- Uranium Hexafluoride Crystal Structure, CCoil – CC BY-SA 3.0 DEED
- Planet Earth – Pexels – c/o Pexels
